© 2024 The authors. This article is published by IIETA and is licensed under the CC BY 4.0 license (http://creativecommons.org/licenses/by/4.0/).
OPEN ACCESS
Wetlands are sites of great ecological and economic importance. However, in Panama, studies that focus on evaluating the health of these ecosystems that constantly face anthropogenic effects are not common. In this work, an analysis of the water quality of farms located in what were coastal wetlands has been carried out, to measure the level of impact and change produced by the anthropological activities carried out and to be carried out in the area of study, located on the coast of Tonosí, Los Santos. For this, the NSF quality index methodology has been used, using variables obtained from 7 sampling plots each with varying numbers of subplots. Within these influences, a variety of emerging contaminants were observed, the greatest variety of these found in plot 2 in particular. The results point to undeniable contamination of the site based on agricultural and livestock activities as the main culprits.
water quality, surface waters, interstitial waters, emerging contaminants, variables, nutrients, plots, Tonosi
1.1 Wetlands and their importance
Wetlands are transition zones between terrestrial and aquatic ecosystems, these are characterized by having little depth [1, 2]. These ecosystems are characterized by being complex due to their dynamics in their physicochemical and biological components with high spatiotemporal variability [3, 4]. Isolated wetlands exist, whose moisture comes from groundwater, but these are rare. Wetlands have ecosystem importance and host great biodiversity. However, its greatest contribution - from a certain point of view - is its carbon reserve capacity [5-7]. Currently, the preservation and restoration of wetlands is one of the most effective alternatives to mitigate climate change, the loss of nurseries for commercial species, the loss of a hydrological buffer, and the acidification of both the oceans and rainfall [4, 8, 9].
In Panama, research on freshwater wetlands is scarce, so the conditions and environmental characteristics of these ecosystems are unknown. The expansion of the agricultural, livestock and urban frontier is modifying the ecological dynamics of wetland areas and their surroundings, causing progressive deterioration and loss of the biodiversity that inhabits them [10] so this Research provides basic information on the ecological status of these wetlands.
1.2 Water quality
Although a great deal of index methods has been used daily, there is not a single method recognized as the best [11]. The classification of water quality is very relative depending on the proposed use of this resource, location and type of resource (salt or fresh water) [12-17]. The quality of water depends largely on factors such as geology, geomorphology, location, as well as the intended use of said water source [12, 17-19]. This water quality can be perceived as being affected directly, indirectly by human actions or interventions [14, 18, 20]. According to Intergovernmental Panel on Climate Change (IPCC), climate change will affect the resource, so it is necessary to carry out constant monitoring to know both the quality and the availability of the resource in the water basins [21, 22].
The National Sanitation Foundation (NSF) water quality index is used in the Central American region, due to its wide range of variables and its flexibility with them [8, 21, 23, 24]. Among some of the investigations using the WQI NSF are: monitoring of water for consumption, monitoring of protected areas, characterization of water quality in recreation areas, among others [8, 24]. For the use of this water quality index, multiple variables are measured (percentage of dissolved O2, fecal coliforms, pH, nitrate, phosphate, temperature variability, turbidity, total solids) [18-20, 25].
In the country, freshwater monitoring is very important due to its use for human consumption and for the Panama Canal, however studies on coastal wetlands and analysis of water quality are scarce [26].
1.3 Project objective
The purpose of this study is to create a baseline that serves as a reference to identify anthropological interventions coming from productive activities such as agriculture and livestock in the coastal wetlands that are located on the transition line between mangroves and dry land, specifically close to the mouth of the Tonosí River, in the Tonosí Region, Los Santos Province in the Panamanian Pacific.
2.1 Location of the study area
According to the study [27], different sampling areas were selected in Tonosí.
Tonosí is located southwest of the Azuero peninsula, in the province of Los Santos, Republic of Panama (Figure 1). The main economic and cultural activities in the region are agricultural, aquaculture and livestock. There are multiple livestock farms that were freshwater wetland areas and that were transformed for agriculture, livestock, among others.
Figure 1. Location of the study area, with sampling points
Source: Wetlands International
2.2 Measuring stations, physical-chemical variables and laboratory analysis
The sampling station were located in the lower basin of the Tonosí River. The samples were taken in February 2023, to perform analyses to measure the following parameters: nitrates, phosphates, fecal coliforms, emerging contaminants (agrochemicals) and physicochemical parameters such as dissolved oxygen, pH, temperature, conductivity and turbidity [26].
The physical-chemical parameters were analyzed in situ with the following equipment: multiparameter probe (OHAUS Waterproof pen meter) to measure pH, suspended solids, water temperature and conductivity, Refractometer (Hanna instruments) to measure salinity, turbidity meter (DELTA OHM), oxygen meter (Milwaukee MW605 MAX) to obtain the percentage of oxygen and dissolved oxygen. For these data collections, three replicates were carried out. The methodology described by methods generated by the Blue Carbon initiative [28] and adapted by the Ministry of the Environment of Panama and UNDP [27] was used, where plots (TON) of size up to 125 meters long by 40 meters wide are worked (Figure 2), with subplots (sup), every 25 meters with a radius seven meters each. The maximum number of subplots was six, however, this varied depending on site conditions (Table 1). Once the subplots within them had been measured, water samples were taken. Surface and interstitial water samples were measured in situ with the aforementioned instruments. To take samples of the interstitial waters, a pipette was used and introduced up to 30 cm deep. As a clarification, surface water is considered to be the mass found above the ground that on average was not greater than 10 centimeters, and interstitial water is considered to be that found within the ground up to 30 centimeters deep.
Figure 2. Design of sampling plots [26]
Table 1. Geographic coordinates of the areas of the sample plots in the coastal wetlands of Tonosí
|
Locations |
Spot |
Geographic Coordinates Datum WGS84 |
|
|
Latitude |
Longitude |
||
|
1. Cañas |
C1-1 |
7.433117 |
-80.2321 |
|
C1-2 |
7.4313 |
-80.2321 |
|
|
2. Cañas |
C2-1 |
7.432574 |
-80.2336 |
|
C2-2 |
7.432612 |
-80.2336 |
|
|
3. Bucaro |
B1-1 |
7.353275 |
-80.3735 |
|
B1-2 |
7.353126 |
-80.3736 |
|
|
B1-3 |
7.352971 |
-80.3737 |
|
|
B1-4 |
7.352838 |
-80.3738 |
|
|
B1-5 |
7.352979 |
-80.3737 |
|
|
4. Bucaro |
B2-1 |
7.353935 |
-80.3753 |
|
B2-2 |
7.353925 |
-80.3755 |
|
|
B2-3 |
7.353787 |
-80.3758 |
|
|
B2-4 |
7.353796 |
-80.376 |
|
|
5. Bucaro |
B3-1 |
7.3557 |
-80.3799 |
|
B3-2 |
7.355581 |
-80.3798 |
|
|
B3-3 |
7.355456 |
-80.3797 |
|
|
B3-4 |
7.355346 |
-80.3795 |
|
|
B3-5 |
7.355244 |
-80.3794 |
|
|
6. Bucaro |
B4-1 |
7.356927 |
-80.3861 |
|
B4-2 |
7.356751 |
-80.3861 |
|
|
B4-3 |
7.356568 |
-80.3861 |
|
|
B4-4 |
7.356402 |
-80.3861 |
|
|
7. Bucaro |
B5-1 |
7.355195 |
-80.388 |
|
B5-2 |
7.35502 |
-80.3881 |
|
|
B5-3 |
7.354846 |
-80.3882 |
|
|
B5-4 |
7.354711 |
-80.3882 |
|
To collect nutrient and coliform samples, amber bottles containing specific reagents for each analysis were utilized. These samples were stored in a cooler maintained at temperatures below 10℃ and transported to the laboratory for processing. For contaminant analysis, specifically agrochemicals, the samples were analyzed at the Laboratory for Analysis of Pesticide Residues in Plants and Plant Products, a national reference government laboratory under the Ministry of Agriculture. Two main analytical techniques were employed: gas chromatography with a triple quadruple mass detector (GC-MSMS) and liquid chromatography with a triple quadruple mass detector (LC-MSMS).
The first method, GC-MSMS, was applied to detect the following agrochemicals: acetochlor, alachlor, aldrin, BHC-Gamma, bifenthrin, cadusafos, clomazone, chlorpyrifos, DDD, DDE, diazinon, alpha-endosulfan, etofenprox, ethoprofos, fenpropidin, hexachlorobenzene, metolachlor, mirex, molinate, pirimicarb, primiphos methyl, propiconazole, tefluthrin, terbufos, terbutylazine, tolclofos methyl, trifluralin, and vinclozolin. The second method, LC-MSMS, targeted the analysis of ametrine, atrazine, azoxystrobin, carbendazim, fenamiphos, fenamiphos sulfone, fenpropimorph, imazalil, imidacloprid, metalaxyl, monocrotophos, propamocarb, propoxur, and triadimefon.
The Environmental and Occupational Hygiene Laboratory, accredited by Panama's National Accreditation Council (CNA), was tasked with analyzing total coliforms and nutrients. For total coliforms, the SM 9223 B measurement technique was used, while phosphate levels were analyzed using the SM 4500 PE/HACH 10210 technique, and nitrates were assessed with HACH 10206. All samples were preserved in amber bottles within a cooler, kept at temperatures below 10 degrees Celsius, with a maximum of eight hours allowed between collection and delivery to the laboratories. Three replicates were conducted for all samples.
2.3 Estimation of indices
The water quality index (WQI) selected for the said analyzes is the NSF (National Sanitation Foundation), using the formula:
$W Q I=\sum_{i=1}^n Q_i W_i$ (1)
In Eq. (1), i represents the multiple variables employed in this WQI, as detailed in Figure 2. The term $Q_i$ denotes the subindex for each variable i, which is determined using standard values from subindex calculation tables. The $W_i$ refers to the weighted value assigned to each variable, specified in Table 2 [8, 12].
Table 2. Weighted felling weights ($W_i$): Custom fabrication
|
Variables |
% Weighted Weight |
|
O2 |
0.17 |
|
Total coliforms |
0.16 |
|
pH |
0.11 |
|
Nitrates |
0.1 |
|
phosphates |
0.1 |
|
turbidity |
0.08 |
|
sun. in suspension |
0.07 |
After obtaining the result of the operation, this value is compared with the table of weighted weights for water quality variables (Figure 3) to determine the contamination level of the sample. The weighted weight represents the significance assigned to each measured variable, with some variables deemed more critical due to their environmental impact. This significance is encapsulated in their weighted weights.
Figure 3. Colorimetric representation of values for water quality
The value table categorizes each level of water quality using a color code ranging from light blue to red, representing quality from excellent to very poor, respectively.
The analysis of physicochemical variables, emerging contaminants, nutrients, and coliforms reveals significant anthropogenic influences from activities such as agriculture, livestock, and urban development. The results for the physicochemical variables are presented in descending order of importance, based on the weighted weights shown in Figure 4. All variables are shown in Table 3 (superficial water) and Table 4 (interstitial).
Figure 4. Surface vs. interstitial dissolved O2 concentration comparison graph divided by plot (Ton) and subplot (sup)
Table 3. Averages of the physicochemical and biological variables of surface water by plot and subplot, in Tonosí
|
Superficial |
|||||||||
|
Physical Chemical Parameter |
Shallow Subplot |
Average t |
Sun. in Suspension |
Ph |
Turbidity |
O2% |
Coliforms |
Nitrate |
Phosphate |
|
Ton 1 |
sup 1 |
28.77 |
349 |
7.61 |
43.07 |
4.17 |
5.67 |
0.9 |
14 |
|
sup 2 |
30.4 |
348.67 |
7.52 |
35.3 |
7.19 |
5.67 |
0.9 |
14 |
|
|
sup 3 |
35 |
337.33 |
7.57 |
21.63 |
5.55 |
5.67 |
0.9 |
14 |
|
|
sup 4 |
34.7 |
393 |
7.32 |
19 |
6.35 |
5.67 |
0.9 |
14 |
|
|
Ton 2 |
sup 1 |
32.73 |
301 |
8.32 |
12.37 |
8 |
13540 |
4 |
13 |
|
sup 2 |
31.37 |
2.72 |
7.82 |
14.77 |
6.89 |
13540 |
4 |
13 |
|
|
sup 3 |
30.3 |
286.67 |
7.77 |
14.37 |
7.97 |
13540 |
4 |
13 |
|
|
sup 4 |
29.5 |
308 |
7.4 |
12.93 |
5.89 |
13540 |
4 |
13 |
|
|
Ton 3 |
sup 3 |
37.07 |
480.33 |
8.61 |
151 |
2.88 |
87040 |
0.9 |
10 |
|
sup 4 |
35 |
481 |
8.41 |
43.5 |
6.51 |
87040 |
0.9 |
10 |
|
|
sup 5 |
32.4 |
602.67 |
7.73 |
11.6 |
4.23 |
87040 |
0.9 |
10 |
|
|
Ton4 |
sup 1 |
27.1 |
497.67 |
7.91 |
42.13 |
7.32 |
111990 |
0.9 |
4.75 |
|
sup 2 |
27.8 |
486 |
7.78 |
88.03 |
3.68 |
111990 |
0.9 |
4.75 |
|
|
sup 3 |
28.9 |
538.67 |
8.31 |
3.66 |
2.12 |
111990 |
0.9 |
4.75 |
|
|
sup 4 |
30.6 |
760.67 |
8.07 |
91.63 |
0.55 |
111990 |
0.9 |
4.75 |
|
|
Ton 5 |
sup 1 |
31.4 |
386.67 |
7.8 |
8.5 |
10.01 |
24810 |
0.9 |
3.26 |
|
sup 2 |
32.1 |
423.67 |
7.9 |
10.77 |
8.76 |
24810 |
0.9 |
3.26 |
|
|
sup 3 |
32.5 |
396 |
7.73 |
22.73 |
5.71 |
24810 |
0.9 |
3.26 |
|
|
sup 4 |
32.4 |
366.33 |
6.34 |
14.81 |
6.82 |
24810 |
0.9 |
3.26 |
|
|
Ton 6 |
sup 1 |
31.4 |
449.67 |
7.21 |
12 |
4.34 |
58000 |
0.9 |
3.2 |
|
sup 2 |
34.4 |
369.67 |
7.43 |
4.06 |
3.84 |
58000 |
0.9 |
3.2 |
|
|
Ton 7 |
sup 1 |
28.2 |
489.67 |
6.65 |
248.33 |
1.67 |
7170 |
5 |
eleven |
|
units of measurement according to variable |
T℃ |
mg/L |
pH |
NTU |
% |
NMP/100ml |
mg/L |
mg/L |
|
Table 4. Average physicochemical and biological variables of interstitial water divided by plot and subplot, obtained in the Tonosí study area
|
Interstitial |
|||||||||
|
Chemical Physical Parameter |
Intercial Subplot |
Average Temperatures |
Sun. in Suspension |
pH |
Turbidity |
O2% |
Coliforms |
Nitrate |
Phosphate |
|
Ton 1 |
sup 1 |
30.50 |
458.00 |
7.21 |
377.60 |
2.77 |
5.67 |
0.9 |
14 |
|
sup 2 |
30.00 |
378.67 |
7.21 |
442.33 |
5.79 |
5.67 |
0.9 |
14 |
|
|
sup 3 |
32.60 |
355.00 |
6.91 |
442.33 |
2.33 |
5.67 |
0.9 |
14 |
|
|
sup 4 |
32.80 |
512.33 |
6.76 |
308.00 |
0.39 |
5.67 |
0.9 |
14 |
|
|
Ton 2 |
sup 1 |
29.6 |
477.67 |
7.25 |
282.00 |
0.63 |
13540 |
4 |
13 |
|
sup 2 |
29.9 |
871.33 |
6.68 |
258.00 |
0.80 |
13540 |
4 |
13 |
|
|
sup 3 |
29.1 |
566.33 |
6.40 |
343.33 |
0.27 |
13540 |
4 |
13 |
|
|
sup 4 |
28.8 |
475.67 |
6.93 |
545.00 |
0.49 |
13540 |
4 |
13 |
|
|
Ton 3 |
sup 3 |
30.6 |
570.00 |
7.31 |
930.33 |
0.54 |
87040 |
<1 |
10 |
|
sup 4 |
30.1 |
604.67 |
7.95 |
473.33 |
0.5 |
87040 |
<1 |
10 |
|
|
sup 5 |
29.8 |
835.00 |
7.10 |
952.67 |
0.24 |
87040 |
<1 |
10 |
|
|
Ton4 |
sup 1 |
27.9 |
589.67 |
7.46 |
530.00 |
1.32 |
111990 |
<1 |
4.75 |
|
sup 2 |
27 |
631.67 |
7.23 |
170.00 |
1.17 |
111990 |
<1 |
4.75 |
|
|
sup 3 |
28 |
596.67 |
7.46 |
815.67 |
0.45 |
111990 |
<1 |
4.75 |
|
|
sup 4 |
29.4 |
466.67 |
7.29 |
542.33 |
0.46 |
111990 |
<1 |
4.75 |
|
|
Ton 5 |
sup 1 |
30.4 |
348.33 |
7.16 |
821.33 |
0.84 |
24810 |
<1 |
3.26 |
|
sup 2 |
29.6 |
586.33 |
7.12 |
802.67 |
0.51 |
24810 |
<1 |
3.26 |
|
|
sup 3 |
30.2 |
541.00 |
6.79 |
860.33 |
0.33 |
24810 |
<1 |
3.26 |
|
|
sup 4 |
28.9 |
406.00 |
6.75 |
570.67 |
0.66 |
24810 |
<1 |
3.26 |
|
|
Ton 6 |
sup 1 |
30.1 |
542.67 |
7.48 |
417.33 |
4.22 |
58000 |
<1 |
3.2 |
|
sup 2 |
30.8 |
480.33 |
7.48 |
672.67 |
2,955 |
58000 |
<1 |
3.2 |
|
|
Ton 7 |
sup 1 |
27 |
485.00 |
6.99 |
313.67 |
1.15 |
7170 |
5 |
eleven |
|
units of measurement according to variable |
T℃ |
mg/L |
pH |
NTU |
% |
NMP/100ml |
mg/L |
mg/L |
|
The concentration of dissolved O2 is the highest in the importance table due to all the factors that can influence it such as temperature, pressure, solubility coefficient, salinity and physicochemical composition of water, therefore, it is a great indicator and comparative variable [1, 8, 22]. The greatest source of oxygenation is the contact of the water surface with the air and temperature is a fundamental factor for the solvency of O2 in water [19, 22] The graph in Figure 5 shows the percentage of O2 low and constant for the interstitial waters, on the other hand in Ton 7 there was almost no water so there was no observable flow and the water temperature was high from what can be seen a low percentage of dissolved oxygen.
pH is a measure of alkalinity/acidity, which is made up of measurements from the number 1 to 14 (in natural substances, but there are non-natural substances or concentrations that can exceed this measurement up or down). The normal pH of water has an average of 7.35 [22]. Anthropological activities have introduced inorganic compounds from livestock and agriculture, which greatly affect the pH level of a water column [15]. Figure 5 shows pH levels with little difference between surface and interstitial waters.
Figure 5. Comparison of the surface vs. interstitial pH level in the study plots
Temperature is one of the most relevant physical parameters for the analysis of water, since it affects other variables such as viscosity and the speed of chemical reactions [15]. The temperature averages obtained show a stable temperature with little variability in the plots. In general, these temperature ranges are normal in the tropical zone and typical of the tropical humid forest (refer to Figure 6).
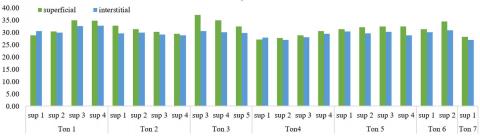
Figure 6. Averages of surface vs. interstitial temperatures in the study plots
Turbidity can be defined as the ability of the solid suspended within a column or tributary of water to block the passage of light. The causes of turbidity are multiple and varied. Among which we can mention: erosion (natural and/or anthropological source) and contamination by direct human activities such as constructions or excavations (anthropological) [15].
Turbidity is closely linked to the level of dissolved solids. Regarding its level in the surface water, we can see a very low level in most of the subplots with some points of rise not exceeding 200 nephelometric turbidity unit (NTU). With the exception of plot 7 where we can see a kind of convergence between the turbidity level of the surface water and the interstitial, due to the low amount of water that was present in the area (Figure 7).
Figure 7. Comparative graph of turbidity of surface vs. interstitial water
Dissolved solids can be defined as any material that remains in a water sample once it is evaporated at more than 105℃, in other words, everything in a water sample that is not water [15]. In our study, the amount of dissolved solids ranged between values of 286.67 and 760.67 mg/L with a marked drop in subplot 2 of plot 3 where there was a more marked water current than in the rest of the study area, on the contrary the rising peak of subplot 4 of plot 4 is due to a marked stagnation of water, so any movement such as sample collection created a rise of solids, Sierra Ramírez [14] ensure that agriculture has an impact in the amount of dissolved solids, however, plots six and seven (agricultural use) in our study present levels similar to or lower than plots for livestock use. On the other hand, we can observe that the level of dissolved solids in the interstitial water is higher on average because, as described in the methodology, these samples were obtained after opening a hole in the wetland soil with the drilling hole. extraction of soil cores (Figure 8).
Figure 8. Comparative graph of suspended solids in surface vs. interstitial water
I. Water quality analysis for nitrate: The maximum amount of nitrate accepted as non-toxic is 50 mg/L to prevent the short-term toxic effect due to the production of methemoglobinemia, the standard established in your state is no more than 10 mg/L [29, 30]. The Complutense University of Madrid, tells us that uncontaminated water is around 4 mg/L, concentrations greater than this number could largely affect ecosystems because they favor an excessive increase in algae [30]. In the analyzes carried out on the plots, values lower than 1 mg/L are evident in four of the seven plots, however, plots 2 and plot 7 present 4 mg/L and 5 mg/L respectively, these values are not considered toxic and can occur naturally, but it should be noted that plot 7 presenting the highest concentration of nitrate has agricultural use by the population, which agrees with what was found in references [9, 14].
II. Phosphates: Under natural conditions, the concentration of phosphates in water is less than 1 mg/L; concentrations greater than this number could largely affect ecosystems because they favor an excessive increase in algae (eutrophication) [6]. The phosphate levels obtained in the samples are worrying and demonstrate a high level of anthropological influences in the sampling area, Sierra Ramírez [14] found results superior to those found in our study area, the study [9] obtained more similar results to ours but they come to the conclusion that these are due to the activities identified as grazing animals, wastewater discharge and cultivation, all of these activities carried out in the area. The study [9] mentions that in the tropics phosphorus is consumed very quickly, therefore the only way in which an increase is noticeable is if there is anthropological intervention. Under normal conditions, as was already clear in the explanation part of each measured compound, the normal level of Phosphates in the environment is less than 1 mg/L, so the effect of anthropological contamination in this first area of the study is undoubted. The highest concentration of phosphates occurs in plot one with a value of 14 mg/L, followed by plots two and seven with concentrations of 13 and 11 mg/L respectively.
Figure 9 shows us the difference in the concentrations of both nutrients, the mostly normal level of nitrate is a clear contrast to the abnormal quantities in the present of phosphates.
Figure 9. Comparison of nitrate and phosphate per plot in the study area, Tonosí
III. Coliform analysis: Coliforms are a type of bacteria which are present in tributaries contaminated by fecal material, which is why they are of significant importance as pollution indicators [21]. The permissible limits established by Water Law 75 for Panama for the recreational use of water sources is ≤250 bacteria/100ml for wastewater), according to the national standards of Costa Rica, the maximum permissible limit is ≤1000 bact/100ml [13]. As is shown in the Figure 10 all sampled plots presented harmfully high bacterial indices ranging from 13,540 bact/100ml to 111,990 bact/100ml; With the exception of plot 1, it had a bacterial presence of 5670 bact/100ml. Researchers [9] found similar results in their sampling, where they highlight that the abundance of this biological marker is due to a discharge of fecal matter. Sierra Ramírez [14] focused on fecal coliforms, but reached the same conclusion. especially giving rise to anthropological activities carried out in the study area. It should be noted that according to Panamanian Commission of Industrial and Technical Standards (COPANIT), all these samples are dangerously harmful if this water is used for human consumption.
Figure 10. Total coliforms detected in the plots in the study area, Tonosí (NMP/100ml = bact/100ml)
IV. Analysis of agrochemicals: Agrochemicals are multiple substances used for different situations of plant or animal pests, whose effects and concentrations will be detailed one by one. Most of the plots presented low but existing concentrations of Chlorpyrifos and Ethoprofos (plots 1, 2, 3 and 7), plot 2 was the most contaminated with the presence of multiple agrochemicals in different concentrations. Plots 4 and 5 did not present any concentration of agrochemicals; It should be clarified that this does not mean that they have not been contaminated, but rather that they were not seen during the sampling period. Even so, it is considered that they may be contaminated. Agrochemicals present in the sampling area [9, 31].
All detected agrochemicals are presented in this table. Within which some results are shown with <0.14 those that were detected, but in smaller quantities than those measured precisely. On the other hand, the “-” show that these agrochemicals were not present in the plots. The table shows us a clear and significant presence of agrochemicals in plot 2.
Chlorpyrifos: synthetic compounds or components not found in the nature. Depending on the amount or time of exposure, both breathing and ingesting these compounds can cause various negative effects on the nervous system, such as headaches, vision problems, seizures with salivation, coma or even death. In the plots in which this agrochemical was present, it occurred in concentrations lower than 0.14 µg/L, which is not considered harmful to human health [9, 32].
Ethoprofos is a synthetic nematicide which is not naturally present in nature. This has a moderate solubility in water and a low resistance in soil. Highly toxic to aquatic organisms. For this study, the concentrations were also low in most cases at 0.14 µg/L, which is not considered harmful [9, 32, 33].
Bifenthrin: it is a synthetic insecticide, it has low solubility in water, but high resistance in the soil, its toxicity is very high in invertebrate organisms such as crustaceans and medium in others such as birds [9, 34]. Its concentration in plot 2 was 0.29 µg/L, which is considered low [18] It should be noted that this was analyzed in water but not in soil, so it may be in higher concentrations than expected.
DDD and DDE: are synthetic compounds that are not present in nature which are derived from the agrochemical Dichlorodiphenyltrichloroethane (DDT), which is prohibited for sale. They are not biodegradable, they have a low solubility in water, but their resistance in soil is considered extreme. They are especially toxic to crustaceans and fish [9, 35]. In Central America and other parts of the world, a concentration of <1 µg/L is considered the maximum allowed to be considered non-toxic. These compounds are presented in the analysis of plot 2. They were present in the analyzes of plot 2 in amounts of 0.193 µg/L of DDD and concentrations <0.14 µg/L of DDE, which is not considered toxic and does not occur in the rest of the plots.
Etofenprox: are synthetic compounds that are not present in nature. Its solubility and resistance in soil are very low. However, they are especially toxic to aquatic organisms [9, 36], the European Union considers the limit of <1 µg/L as non-toxic. In our study it was found in plot 2 with a concentration of <0.14 µg/L in plot 2.
Metolachlor: synthetic herbicides that do not occur in nature. Highly toxic to aquatic organisms, its solubility is high and its resistance in the soil is low [9]. Concentration in plot 2 (the only plot in which it was present according to the analysis) of <0.14 µg/L, somewhat lower than the maximum set by Holland of <0.2 µg/L.
Mirex: Low water solubility and zero soil resistance. It is a compound present in multiple types of pesticides [9]. Its concentration in plot 2 (the only plot in which it was present according to the analysis) is <0.14 µg/L.
Pirimicarb: Synthetic pesticide compound with high solubility and extreme soil resistance. High toxicity for aquatic organisms [9, 25]. Maximum limits in Europe are <0.9 µg/L. Concentration in plot 2 (the only plot in which it was present according to the analysis) of <0.14 µg/L.
Terbuthylazine: synthetic herbicide compound, which solubility is low and its resistance in the soil is extreme. It is present in a high number of pesticides and according to European standards a maximum of <0.9 µg/L is considered [9]. High toxicity for aquatic organisms. Concentration in plot 2 (the only plot in which it was present according to the analysis) of <0.14 µg/L.
As we have observed, the two most frequently found agrochemicals with the clear exception of plots 4 and 5 are chlorpyrifos and ethoprofos which were explained at the top of the document (Table 5). Plot 2 was the one with the greatest abundance of agrochemicals, but their actual concentrations (for many) were lower than what the laboratory equipment could measure. Furthermore, many of these agrochemicals, as mentioned in their explanations, have low solubility and high resistance in the soil, so it is not clear if these were used recently in low concentrations or if they were used a long time ago in high enough concentrations. enough to be measured to this day.
Ethoprofos: The most worrying group of chemicals, their presence in plot 6, although notable, does not have a concentration high enough to be dangerous, plots 3 and 7 where concentrations of 224 and 221 respectively are seen, which is considered as they can be toxic. These concentrations are much higher than what is accepted (1 µg/L) for human use and contact in most countries.
Figure 11. Comparative graph of WQI values per surface vs interstitial plot
Table 5. Agrochemicals present in each plot of Tonosí
|
Agrochemicals |
TON 1 |
TON 2 |
TON 3 |
TON 4 |
TON 5 |
TON 6 |
TON 7 |
Units |
|
Chlorpyrifos |
<0.14 |
<0.14 |
<0.14 |
- |
- |
0.187 |
<0.14 |
µg/L |
|
Ethoprofos |
<0.14 |
<0.14 |
224 |
- |
- |
0.283 |
221 |
µg/L |
|
Bifenthrin |
- |
0.29 |
- |
- |
- |
- |
- |
µg/L |
|
DDD |
- |
0.193 |
- |
- |
- |
- |
- |
µg/L |
|
DDE |
- |
<0.14 |
- |
- |
- |
- |
- |
µg/L |
|
Etofenprox |
- |
<0.14 |
- |
- |
- |
- |
- |
µg/L |
|
Metolachlor |
- |
<0.14 |
- |
- |
- |
- |
- |
µg/L |
|
Mirex |
- |
<0.14 |
- |
- |
- |
- |
- |
µg/L |
|
Pirimicarb |
- |
<0.14 |
- |
- |
- |
- |
- |
µg/L |
|
Terbuthylazine |
- |
<0.14 |
- |
- |
- |
- |
- |
µg/L |
Table 6. Results of the WQI calculation of surface water by plot and subplots in the Tonosí study area
|
Parcel/Subplot |
TON 1 |
TON 2 |
TON 3 |
TON 4 |
TON 5 |
TON 6 |
TON 7 |
|
Use of the Plot |
Cattle Raising |
Cattle Raising |
Cattle Raising |
Cattle Raising |
Cattle Raising |
Agricultural |
Urban/Agricultural |
|
Sub 1 |
40.365 |
39.889 |
38.36 |
44.23 |
42.23 |
30.5 |
|
|
Sub 2 |
41.964 |
43.305 |
35.1 |
42.605 |
|||
|
Sub 3 |
42.54 |
41.97 |
38.755 |
39.335 |
42.99 |
||
|
Sub 4 |
42.65 |
40.025 |
44.025 |
39.117 |
39.247 |
||
|
Sub 5 |
48.59 |
||||||
|
Sub 6 |
45.47 |
||||||
|
General |
41.22 |
42.105 |
44.104 |
35.34 |
43.271 |
43.89 |
30.5 |
Table 7. Results of the calculation of WQI of interstitial water by plot and subplots in the Tonosí study area
|
Parcel/Subplot |
TON 1 |
TON 2 |
TON 3 |
TON 4 |
TON 5 |
TON 6 |
TON 7 |
|
Use of the Plot |
Cattle Raising |
Cattle Raising |
Cattle Raising |
Cattle Raising |
Cattle Raising |
Agricultural |
Urban/Agricultural |
|
Sub 1 |
36.715 |
33.187 |
32.865 |
37.185 |
35.61 |
30.5 |
|
|
Sub 2 |
37.593 |
31.564 |
33.545 |
33.245 |
|||
|
Sub 3 |
36.88 |
29.727 |
42.302 |
31.017 |
34.511 |
||
|
Sub 4 |
33.8263 |
30.224 |
41.362 |
33.137 |
34.677 |
||
|
Sub 5 |
42.162 |
||||||
|
Sub 6 |
36.55 |
||||||
|
General |
36.44 |
31.564 |
42.079 |
33.074 |
34.754 |
35.87 |
30.5 |
Note: For color quality, refer to Figure 3
3.1 Analysis of the water quality index
To assess water quality, the NSF WQI was utilized, employing common, easily measurable physicochemical parameters to evaluate the impact of anthropogenic activities on the wetland. The parameters used included water temperature, total coliforms (substituting for fecal coliforms), suspended solids, pH, turbidity, and dissolved oxygen percentage.
The analysis of WQI results across the study area revealed uniformly poor ratings for the Tonosí coastal wetlands across all plots. Specifically, the results from plot 7, which had low water levels, may not accurately reflect the full extent of contamination due to its limited sample size. This study links the usage of each plot and the resultant anthropogenic impacts to the observed levels of contamination and the evident degradation of the wetlands. As depicted in Figure 11, the WQI behavior can also be attributed to seasonal factors such as water scarcity and higher temperatures during the dry season, which typically result in reduced water flow, increased turbidity and dissolved solids, and decreased oxygen levels.
The NSF WQI focuses on basic physicochemical and biological parameters, it does not take into account other influences such as emerging contaminants or phosphates and nitrates for its evolution, however, as has been made clear throughout the study that the influence of anthropic activities on water quality in the Tonosí wetlands cannot be denied.
The poor quality of the water is a reflection of bad practices in the management of anthropological activities that take place around the study area. The principal variables that influenced the WQI result to be “bad” are: the high amount of phosphates, extremely low level of oxygen and the very high amount of coliforms.
The inappropriate use of agrochemicals is seen in the results obtained in which their presence was detected either for the control of pests or herbicides or in the nutrient levels recorded in the waters.
This study provides the base to compare further studies of water quality in fresh water costal wetland in Panama. We recommend the elaboration of a WQI that includes the presence of agrochemical components for application in this type of cases.
This paper was supported by the National Secretariat of Science, Technology and Innovation of the Republic of Panama (SENACYT) (Grant No.: IDDS22-42 169-2022). The project received support from MIAMBIENTE's Regional Office in Los Santos, specifically from Margarito Moreno, Yer Estuard, Teofilo Quintero, as well as from Alfredo Escudero, cattle rancher and land owner who permitted site sampling on his property.
[1] Amado Alvarez, J., Rubiños Panta, E., et al. (2006). Indice de calidad del agua en la cuenca del río Amajac, Hidalgo, México: Diagnóstico y Predicción. Phyton (Buenos Aires), 75: 71-83.
[2] Mitsch, W.J., Gosselink, J.G., Zhang, L., Anderson, C.J. (2009). Wetland Ecosystems. John Wiley & Sons.
[3] Verhoeven J.T.A., Setter, T.L. (2010). Agricultural use of Wetlands: Opportunities and Limitations. Annals of Botany, 105(1): 155-163. https://doi.org/10.1093/aob/mcp172
[4] Ballesteros, E., Romero, J. (1988). Zonation patterns in tideless environments (Northwestern Mediterranean): looking for discontinuities in species distributions. Investigacion Pesquera (Spain), 52(4): 595-616.
[5] Hernández, M.E. (2010). Suelos de humedales como sumideros de carbono y fuentes de metano. Terra Latinoamericana, 28(2): 139-147.
[6] Mitsch, W.J., Bernal, B., Nahlik, A.M., Mander, Ü., Zhang, L., Anderson, C.J., Jørgensen, S.E., Brix, H. (2013). Wetlands, carbon, and climate change. Landscape Ecology, 28: 583-597. https://doi.org/10.1007/s10980-012-9758-8
[7] Kayranli, B., Scholz, M., Mustafa, A., Hedmark, Å. (2010). Carbon storage and fluxes within freshwater wetlands: A critical review. Wetlands, 30: 111-124. https://doi.org/10.1007/s13157-009-0003-4
[8] Gutiérrez Puetate, S.E. (2019). Evaluación espacio temporal del río monjas, sector pomasqui y san antonio de pichincha mediante ICA-NSF. Universidad Central del Ecuador.
[9] MacBean, C. (2012). The Pesticide Manual: A World Compendium. Cambridge, BCPC.
[10] Paytan, A., McLaughlin, K. (2007). The oceanic phosphorus cycle. Chemical Reviews, 107(2): 563-576. http://doi.org/10.1021/cr0503613
[11] Marselina, M., Wibowo, F., Mushfiroh, A. (2022). Water quality index assessment methods for surface water: A case study of the Citarum River in Indonesia. Heliyon, 8(7): e09848. https://doi.org/10.1016/j.heliyon.2022.e09848
[12] Jiménez, M.A., Vélez, M.V. (2006). Análisis comparativo de indicadores de la calidad de agua superficial. Avances en Recursos Hidráulicos, (14): 53-69.
[13] Riđanović, L., Terzić, R., Riđanović, S. (2018). The role of faecal coliforms in water quality assessment. Journal of Survey in Fisheries Sciences, 45-51. https://doi.org/10.17762/sfs.v5i1.165
[14] Sierra Ramírez, C.A. (2011). Calidad del Agua-Evaluación y Diagnóstico. Medellín: Universidad de Medellín.
[15] Lumb, A., Sharma, T.C. Bibeault, J.F. (2011). A Review of genesis and evolution of Water quality index (WQI) and some future directions. Water Qual Expo Health, 3: 11-24. https://doi.org/10.1007/s12403-011-0040-0
[16] Perez, R., Riveiro, F., Jimenez-Nod, M., Mangianello, L., Vega, C., Cova, R., Moreno, J. (2017). Water quality assessment in a Caribbean saltwater wetland. Revista Ingeniería UC, 24(3): 417-427.
[17] Carpio Torres, D.E., Uguña Minchala, R.X. (2022). Determinacion de la calidad de agua en las juntas administradoras de agua potable pertenecientes a la microcuenca del burgay. Bachelor thesis, Universidad Politecnica Salesiana Sede Cuenca.
[18] Sandoval Gío, J.J., Castillo Sánchez, L.E., Zarza Meza, E.A., Hernández Jiménez, J.M., Fernández Serrano, J.H., Pineda Doporto, A. (2018). Toxicidad aguda diferencial de TALSTAR® (bifentrina) y BIOTHRINE® (deltametrina) en la tilapia nilótica Oreochromis niloticus. Revista Internacional de Contaminación Ambiental, 34(1): 45-55. https://doi.org/10.20937/rica.2018.34.01.04
[19] Espejo Cruz, M.E. (2017). Determinación de la calidad fisicoquímica del agua del Humedal el Juncal y su reconocimiento como ecosistema estratégico dentro de la educación básica primaria. Master's thesis, Universidad de Bogotá Jorge Tadeo Lozano.
[20] Vidal, R., Martínez, F., Ayza, M. (1994). Aplicaciones de los modelos de calidad en la simulación de las redes de distribución de agua potable. Ingeniería del Agua, 1(3): 55-68. https://doi.org/10.4995/ia.1994.2644
[21] Solís, J.A.R. (2020). Análisis de sensibilidad de la ecuación aditiva y multiplicativa, utilizadas para comprobar el índice de calidad de agua: Caso del hidrotopo del Río Mensabé. Revista Colegiada de Ciencia, 2(1): 26-42.
[22] Romero Pérez, R., López Mera, R., Martínez Ruiz, J. L., López Ramírez, E., Millán Malo, G., Soares Moraes, D. (2018). Estudio de la factibilidad ambiental, desarrollo sustentable, urbano, social y legal para el desarrollo de estrategias participativas y de mediación social para la construcción de sistemas de humedales artificiales (SHA) para el saneamiento del aporte del Río Amanalco a la Presa Valle de Bravo. http://hdl.handle.net/20.500.12013/2220.
[23] Carol, E., del Pilar Alvarez, M., Candanedo, I., Saavedra, S., Arcia, M., Franco, A. (2020). Surface water–groundwater interactions in the Matusagaratí wetland, Panama. Wetlands Ecology and Management, 28(6): 971-982. https://doi.org/10.1007/s11273-020-09762-9
[24] Robledo-Hernández, J.A. (2022). Evaluation of the ICA-NSF water quality index in the micro-basins of the Río Dulce National Park as a tool in the comprehensive management of sustainable management, Livingston, Izabal, Guatemala, Central America. Tecnología en Marcha, 36(1): 106-116. https://doi.org/10.18845/tm.v36i1.6241
[25] EPA. Health and Environmental Research Online: A database of scientific studies and references. https://hero.epa.gov/.
[26] AMBIENTAL, C.G.D.P. (2017). Ministerio del Ambiente. Norma de calidad ambiental y de descarga de efluentes al recurso agua.
[27] Sempris, E., Pinzón, Z., Davis, H.R. (2017). De Medgoondecabbonu Leoosistemls De Manglabes Enlos Distriios Lieismedios Sanf Fuxx y Ministerio De Ambiente, San Lorenzo. Provingade Chirigu.
[28] Howard, J., Hoyt, S., Isensee, K., Telszewski, M., Pidgeon, E. (2014). Coastal blue carbon: Methods for assessing carbon stocks and emissions factors in mangroves, tidal salt marshes, and seagrasses. Arlington, International Union for Conservation of Nature, Switzerland.
[29] Washington State Department of Health. (2016, julio). Nitratos en el agua potable. United Nations Educational, Scientific and Cultural Organisation.
[30] Silva, A.R.E., Cobelas, M.Á., González, E.M. (2017). Impactos del nitrógeno agrícola en los ecosistemas acuáticos. Ecosistemas, 26(1): 37-44.
[31] Ebrahimpour, K., Ebrahimi, A., Baram, M.M. (2023). Investigating the Residue of Diazinon, Chlorpyrifos, and Dichlorvos in urban drinking water supply sources and determining the water quality index in Tirano-Korun in 2020. Journal of Environmental Health and Sustainable Development, 8(4). http://doi.org/10.18502/jehsd.v8i4.14441
[32] Jones, C., Ugalde, M.D.R., Jiménez, K., Mena, F., Vargas, S. (2023). Sensibilidad de Hydra attenuata e Hydra viridis a los plaguicidas diuron y etoprofos, Costa Rica. Uniciencia, 37(1): 542-561. https://doi.org/10.15359/ru.37-1.29
[33] Mena Torres, F., Pfennig, S., Arias Andrés, M.D.J., Márquez-Couturie, G., Sevilla, A., Protti Q,C.M. (2012). Acute toxicity and cholinesterase inhibition of the nematicide ethoprophos in larvae of gar Atractosteus tropicus (Semionotiformes: Lepisosteidae). Revista de Biología Tropical, 60(1): 361-368.
[34] Yang, Y., Wu, N., Wang, C. (2018). Toxicity of the pyrethroid bifenthrin insecticide. Environmental Chemistry Letters, 16: 1377-1391.
[35] Turusov, V., Rakitsky, V., Tomatis, L. (2002). Dichlorodiphenyltrichloroethane (DDT): Ubiquity, persistence, and risks. Environmental Health Perspectives, 110(2): 125-128. https://doi.org/10.1289/ehp.02110125
[36] Benli, A.C.K. (2015). The influence of etofenprox on narrow clawed crayfish (Astacus leptodactylus Eschscholtz, 1823): Acute toxicity and sublethal effects on histology, hemolymph parameters, and total hemocyte counts. Environmental Toxicology, 30(8): 887-894. https://doi.org/10.1002/tox.21963.